TheraLife Publication
Oral TheraLife® Eye Products: Efficacy and Safety in the Treatment of Dry Eye Disease
Todd Severin, MD1, Ron Seger, OD2, Don Robinson, OD3, Yuanjin Tao, OMD L.Ac4
Objective: To compare the efficacy and safety of TheraLife Eye Formula plus TheraLife Enhancer to placebo in patients with moderate eye disease.
Design: Randomized, multi-center, double blind, placebo-controlled trial.
Participants: Total Patient enrollment: 83 (treated 54),(placebo 29)
Intervention: Patients ingested twice daily two TheraLife Eye capsules and two TheraLife Enhancer capsules during week one and two, followed by two TheraLife Eye capsules only twice daily during week 3 and 4. Control group received placebo capsules.
Main Outcome Measures- Efficacy: Tear breakup time, zone quick, rose bengal staining, injection, and contact lens wear and overall ocular discomfort symptoms
Safety: Occurrence of adverse activity.
Results: Treatment with ThreaLife Eye plus ThreaLife Enhancer formulae produced the most significant objective improvements in tear breakup (p<0.0032) and zone quick test (p<0.003). Among the subjective measures the most significant improvements were burning (p<0.02); dryness (p<0.001); tiredness (p<0.001); contact lens discomfort (p<0.012) and overall ocular discomfort (p<0.005). Global assessment by both investigators and patients gave significantly greater improvements in the treatment group (p<0.001) versus the placebo group. TheraLife Eye Formula plus TheraLife Enhancer exhibited excellent safety without significant adversity.
Conclusion: The noval oral ophthalmic formulation were safe and effective in treatment of moderate dry eye disease yielding improvements in both objective and subjective measures. TheaLife oral formulations may provide significant patient benefits with dry eye diseases.
- Todd Severin, MD – East Bay Eye Center, Albany and San Ramon California
- Ron Seger Family Eye Practice, Mountain View, California
- Optometry at Redwood Shores, Redwood City, California
- TheraLife® Inc. Los Altos, California
Headline: TheraLife® Eye Products in the Treatment of Dry Eye Diseases.
The oral administration of TheraLife® Eye ophthalmic formulations was tested in patients with moderate dry eye disease. The results demonstrate statistically significant clinical improvements.
There are many factors that cause dry eyes, including but not limited to: age, allergy, autoimmune diseases, certain medications, chronic diseases, environmental pollutants, indoor air quality, overuse of computers, pre-and postmenopause, and wind.
The most common treatment for dry eyes is teardrops, which gives temporary relief. The disadvantage is that teardrop moisture is lost quickly, requiring multiple applications per day. Soft contact lens wearers in particular experience rapid evaporation of tears from the eye, causing irritation, protein deposits, infection, and pain.
A multivitamin and mineral supplement with basic eye-specific nutrients is known 1. The results are generally vague and relief comes slowly over many years. There are current products on the market that use various oils, such as linseed oil, and fish oil to lubricate the mucosal system in general, including dry eyes. Most of these methods are considered temporary and the dry eye patients really want a convenient product that can sustain comfort throughout the day without frequent reapplication. This was the challenge for TheraLife® to develop the – TheraLife® Eye plus TheraLife® Enhancer formulae. TheraLife® Eye plus Enhancer products are easy to administer (oral capsules) and provide sustained relief through a combination of herbal extracts, vitamins, and minerals. The strategy rests on three principles: (i)Reconstitution of the tear film architecture by correcting compositional imbalances. This is achieved by activities in the herbal extract, which support the metabolism and circulation of the underlying cellular structures responsible for the production of these fluid components. (ii)Prevention of injurious agents to take hold, e.g., neutralizing free radicals and other oxidative elements, by means of antioxidants in the formulations. (iii) Intercepting the inflammatory cascade by immuno-modulating ingredients with anti-inflammatory activity present in the TheraLife® ophthalmic formulation.
The objective of this study was to compare the efficacy and safety of TheraLife Eye formula plus TheraLife Enhancer to placebo in patients with moderate eye disease.
Materials and Methods
Study Design: Six optometric clinics were involved in this randomized, parallel-group double-masked, clinical trial comparing treated subjects with placebo who received only rice powder capsules. The subjects were randomly assigned by a statistician either to the treatment (2/3 of subjects) or the placebo group (1/3 of subjects.). A total of 83 subjects were recruited of which 54 were treated and 29 were placebo. The treatment phase was 4 weeks followed by a one-week post therapy interview. The results of the six sites were combined for analysis.
The Institutional Human Subject Review Committee subjectively reviewed the clinical trial protocol at HRS. All patients signed a prescreen informed consent form, and had a detailed medical history record prior to the study.
Patient Population: Adult patients, from 21 to 65 years old, computer users (average of 3 hrs per day) with vision 20/20 or 20/40, either sex were eligible for participation if they had a diagnosis of moderate dry eye disease as defined by the following criteria (a detailed descriptions of each are given under Outcome Measures: (1) Zone Quick test should be minimum of 5 mm in 15 seconds. (2) Tear Breakup test should be nimum 5 seconds. (3) A score of ≥ 3 on the subjective parameters must have been present despite conventional management, which may have included artificial tear drops, gels and ointments.
Patients were excluded from the study if they were breast-feeding or pregnant females; had a history or clinical evidence of significant chronic or uncontrolled concomitant illness; eye injurys; eye surgery; or were drug abusers within last five years. Use of any medication causing dry eyes within two weeks prior to the study; use of any new or investigational as drug within the past 30 days; improper prescriptions for glasses or contact lenses, history of migraine headaches also lead to exclusions.
All raw materials for TheraLife® products are manufactured under current Good Manufacturing Practice (cGMP) in compliant factories in the United States (Qualiherb, Cerritos, CA; Mayway, Oakland, CA). The final finished products (in the form of capsules) are also manufactured under current Good Manufacturing Practice (cGMP) by factories in United States (Acta Pharmaceutical, Sunnyvale, CA).
Concomitant Medication: Any therapy considered necessary for the maintenance of patient welfare was given at the discretion of the investigator and noted on the case report form. If the medication did not interfere with the response to the study medication, the patient was kept in the study. During the study all concomitant medication treatment regimens were kept as constant as permitted by accepted medical practice. Systemic and
topical ophthalmic medication that could interfere with the response to the study medication or the interpretation of the study results was prohibited during the study. This included immunomodulatory agents, anti-depressants, decongestants, antihistamines, blood pressure medication, diuretics, ulcer medication, tranquilizers, and beta-blocking agents.
Study Protocol: Both the treatment group and the placebo group were carried in the study for 4 weeks. A one week post treatment interview was conducted in week 5. During the treatment course, each subject took twice daily two TheraLife Eye capsules and two TheraLife Enhancer capsules during week one and two, followed by two TheraLife Eye capsules only twice daily during week 3 and 4. Control group received placebo. A subset of subjects (23) and investigators (3) was asked to fill out a global assessment questionnaire on week post treatment. During the Treatment phase, patients return for evaluation after week 1, 2, 3 and 4 of treatment. A week after final treatment (5th week), patients also returned for evaluation.
Outcome Measures
Efficacy: The objective signs monitored were the tear breakup test, zone quick test, corneal staining, Injection and rose bengal test. The subjective endpoints use the rating scale of 1 to 10 for symptoms of dry eyes. Investigators and patient’s evaluations of global response to treatment one-week post therapy are compiled as global assessment to treatment success. All these variables were evaluated at baseline. This global assessment used a 4-point scale in which 0 = condition worsened, 1 = condition unchanged, 2 = condition improved and 3 = condition significantly improved. Or cleared.
Zone Quick Test: The cotton thread tear test was performed over 15 s. This test was repeated after 10 min an average of the two results were taken. Care was taken to avoid reflex tearing by practice placement of the thread. If obvious reflex tearing resulted immediately after thread placement, the test was discontinued and repeated after 10 min.2.3
Corneal Staining: The entire cornea was examined using slit-lamp evaluation with a yellow barrier filter and cobalt blue illumination. Staining was graded using the Oxford Scheme 6-points scale (from 0 -5),
Injection: A subjective assessment of the inflammation of the conjunctiva.
Rose bengal test: One drop of rose bengal 1% (Chauvin) was instilled to the inferior conjunctiva sac and the subject was instructed to blink several times to distribute the stain. The van Bjisterveld staining score was used.4
Symptoms of ocular discomfort that included dryness, redness, burning, itching, tearing, blurry vision, pain or soreness, sandy (gritty), contact lenses discomfort, tired or eyestrain, overall ocular discomfort, were graded using 1 to 10 analog scale (1 being no symptom). A negative change from baseline indicated an improvement.
Safety. The primary safety variable monitored was the occurrence of adverse events. The investigator as none, unlikely, possible, probable, or definite, assessed the relationship of the event to the study medicine. Safety variables were evaluated at baseline and at all study visits. Side effects were reported back to TheraLife®. Todd Severin MD, a licensed Ophthalmologist, served as the medical officer for the study.
Statistical Methods – Tood, we need your input on how much statistics is necessary. Our revision starts again at results.
Our results demonstrate clinical symptom improvement in the treatment of dry eye diseases using the following paratmeters; _____________________ All analyses presented in this report were analized by using the S-Plus 6 software from Insightful Corp.
1998-2001.
conducted on the treatment patient population (all patients randomized variables were collected on both eyes and data from the both eyes were included in the analyses. All safety analyses included data from both eyes.
Descriptive statistics were used to summarize all continuous variables (such as tear break-up time) and categorical variables (such as symptoms of eye discomfort scales).
Repeat measures analyses of variance were used to analyze the scores on each of the 12 subjective and 5 objective measures of eye discomfort. The two repeated factors were time (baseline, week 1, week 2, week 3, week 4) and eye (left/right). The fixed factor was group (treatment/placebo). See Table 1.
Also the Two-sample t test was used to analyze between the treatment and placebo group with respect to changes from baseline in continuous variables; the two side Wilconox rank sum test was used to analyze between the treatment and placebo groups with respect with respect to change from baseline in categorical variables.
Chi-square test was used to analyze global assessment.
For 80% power to detect real differences in this study with a one-tailed 5% Type 1 error rate, with the test performed as noted above, an effect size of 0.60 was required. This is equivalent to the requirement that a random subject receiving agents has a probability of 0.63 of having a better response to therapy than a subjective receiving placebo.
A two-sided test with a p value ≤ 0.05 was considered statistically significant.
Patient Treatment Assignment
Qualified patients were randomly assigned to receive treatment medication or placebo in a 2: 1 ratio using a block size of 2. Only a single randomization was used per trial.
Study Masking
Both the active and placebo capsules were coded by the Health Research and Study Center, and blinded to the physicians, subjects and TheraLife personnel.
Results
Patient Demographics
Our analysis shows no statistical difference between age, sex and race amongst the study population.
Efficacy Analysis
Scores on each of the 12 subjective and 5 objective measures of eye discomfort were subjected to repeat measures analyses of variance. The two repeated factors were Time (baseline, week 1, week 2, week 3, week 4) and Eye (left/right). The fixed factor was Group (treatment/placebo). See Table 1.
No statistically significant of results were found between the right and left eyes, over the study period for the two groups and the four time periods (weeks 1-4).
The Two-sample t test was used to analyze between treatment and placebo groups with respect to changes from baseline in continuous variables( Tear Breakup Test and Zone Quick Test); the two side Wilcoxon Rank Sum test was used to analyze between treatment and placebo groups with respect to change from baseline in categorical variables (Dryness, Burning eye, Contact lens discomfort, Tired eye and overall discomfort). The intent-to treat data set was used in this analysis. Results are shown in Table 1.
Table 1: Statistical Variance by Group at End Point | |
| Subjective Dimensions (Wilcoxon Rank Sum Analysis) | p (week 4) |
Dry Burning Contact Lens Discomfort Overall Discomfort Tiredness
| £0.001 £0.020 £0.012 £0.005 £0.001
|
| Objective Dimensions (Two Sample t- test Analysis) | p (week 4) |
Tear Breakup Test Zone Quick Test | £0.003 £0.003 |
Global Assessment:
Both subjects in the study and optometrists also indicated at the end of the study whether they thought the participants condition showed no improvement, improvement, or cure. This was done by a subgroup composed of 27 participants and 3 optometrists. The self-assessments results are shown in Table 2.
| Table 2. Global Assessment by Subjects and Clinical Investigators | ||||
| Subject | Clinical Investigators | |||
| Improved | Treatment | Placebo | Treatment | Placebo |
| No | 1 | 6 | 0 | 7 |
| Yes | 17 | 3 | 17 | 3 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Theralife® Eye and Enhancer provided a statistically significant improvement for the tear breakup test, zone quick test, dryness, burning, contact lens discomfort, tiredness and overall eye discomfort compared to the placebo.
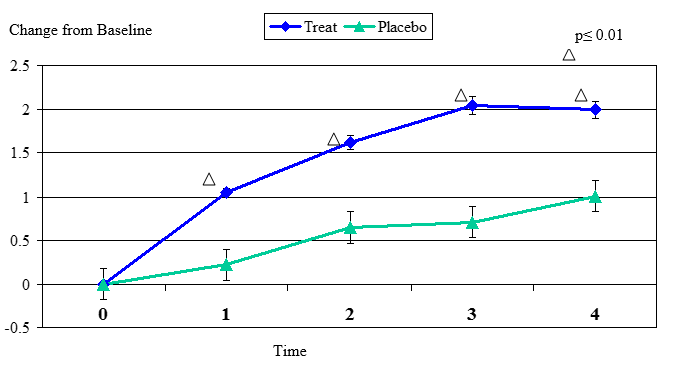
Figure 1. Change From Baseline for Tear Breakup Test
Tear Breakup Test change from baseline. Mean value ± standard error. The statistically significant improvement is within first week.
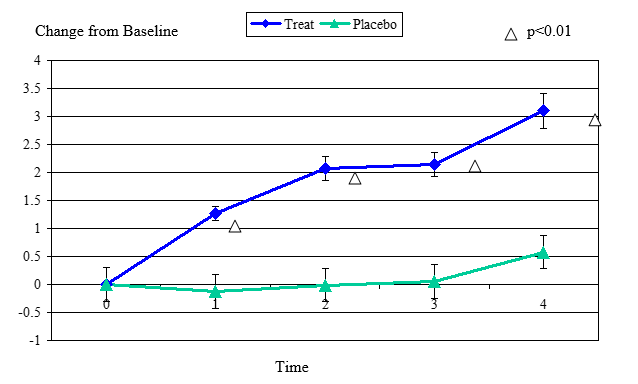
Figure 2 Change From Baseline for Zone Quick Test
Zone Quick Test change from baseline, mean value ± standard error. The statistically significant improvement is within first week, p≤ 0.01.
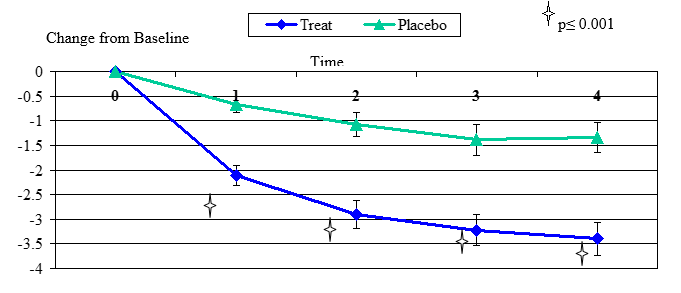
Figure 3. Change From Baseline for Dryness
Dryness change from baseline, mean value ± standard error. The statistically significant improvement is within first week, p≤ 0.001.
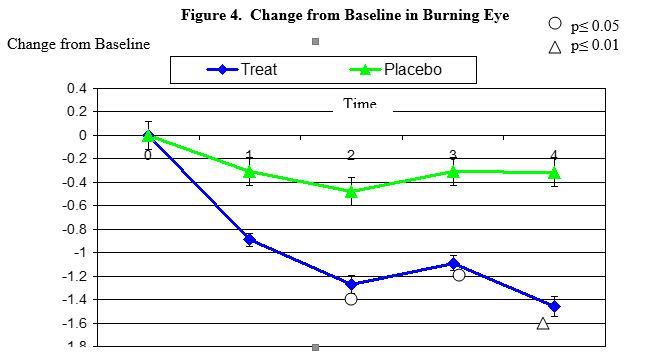
Burning secession changes from baseline. Mean value ± standard error. The statistically significant improvement is within second week, p≤ 0.05, within fourth week p≤ 0.01
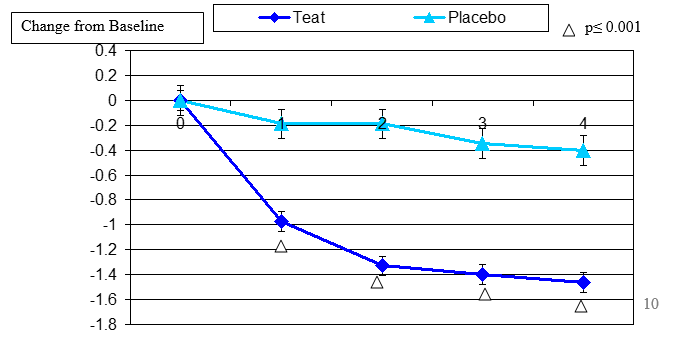
Figure 5. Change From Baseline for Contact Lens Discomfort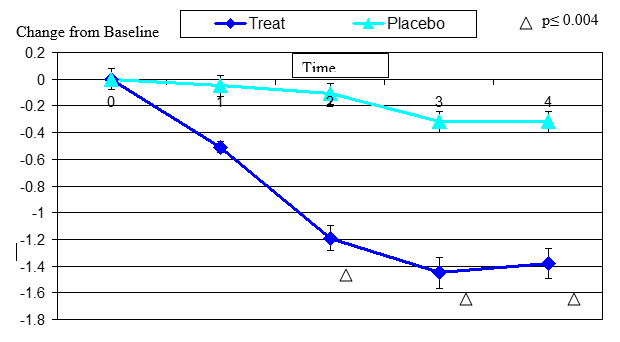
Contact lens discomfort change from baseline. Mean value ± standard error. The statistically significant improvement is within second week, p≤ 0.05.
Figure 6. Change From Baseline in Tiredness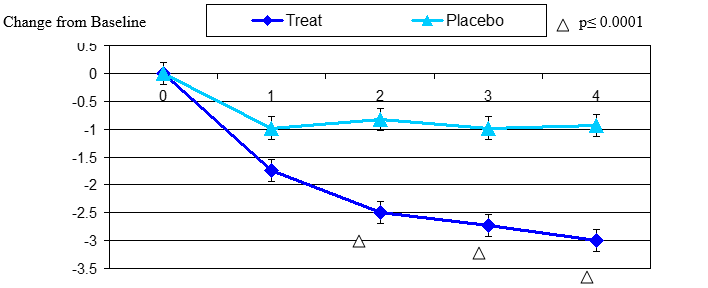
Tired eye change from baseline. Mean value ± standard error. Mean value ± standard error. The statistically significant improvement is within second week, p≤ 0.0001.
Figure 7. Change From Baseline in Overall Eye Discomfort
Overall eye discomfort change from baseline. Mean value ± standard error. The statistically significant improvement is within first week, p≤ 0.001.
Adverse Events and Report
One patient dropped out during the study due to upset stomach in the treatment group.
Discussion
The most significant result of this study conclusively demonstrates Theralife® Eye and Enhancer formulae significantly improved the ocular symptoms of moderate dry eye condition.
We find statistically significant improvements in the objective parameters (tear breakup and zone quick tests) and in the primary subjective symptoms (burning sensation, dryness, eye fatigue, contact lens discomfort overall eye discomfort) in the treatment versus the placebo group in a 4 week study of moderate dry eye condition. Subjective symptoms for dryness and overall eye discomfort and objective parameters for tear breakup and zone quick showed a statistically significant difference from placebo by first week, with further improvement by week two . Based on these study results the conclusion is drawn: Theralife® Eye with Enhancer provide relief from the signs and symptoms of dry eye and eye fatigue within one week of oral treatment.
The combination of patient subjective improvement, physician documentation and objective corroboration are the net result of TheraLife® Eye and Ehnacer formulations, consisting of herbal extracts, vitamins and minerals. The improvements in objective measures strongly suggest that the subjective improvements are resulting from a resolution of the underlying pathophysiology of dry eye manifestations and are not merely symptomatic in nature.
The study found Theralife® Eye and Enhancer have an excellent safety profile. This confirms that the herbal extracts combined with vitamins & minerals contained in Theralife® Eye plus Enhancer formulae are well tolerated..
It is important to keep in mind that there is currently no oral therapeutic treatment for dry eye disease and eye fatigue. The only treatments available are topical and palliative in nature, and provide insufficient relief for many patients, particularly those with chronic moderate symptoms acquired among computer users. These patients are at increasing risk of ocular surface damage and ocular infection, as well as subject to chronic ocular discomfort and visual difficulties. The data presented demonstrates improvement in objective and subjective measures of dry eye disease exceeding expectations with other existing treatments. Moreover, the established change in objective measures suggests the possibility that treatment with Theralife® Eye plus Enhancer may also result in a clinically significant change in the progression of the disease and its consequence.
Conclusions: The noval oral ophthalmic formulation were safe and effective in treatment of moderate dry eye disease yielding improvements in both objective and subjective measures. TheaLife oral formulations may provide significant patient benefits with dry eye diseases.
Acknowledgement:
Burton Worrell, OD4, Gregory Tom, OD5, Lily Yang, PhD7, Somi Oh, OD8
Joann Liddicoat OD9,Gene Spiller, PhD10. ,Judy Turkalj MD
Special thanks to Dr. William Ron Coughlin and ___________ for review of the manuscript.
Reference:
_____________________
1 MaculaRx & MaculaRx Plus” manufactured by ScienceBased Health corporation, Carson City, NV.
2 van Bijsterveld OP. Diagnostic test in the Sicca syndrome. Arch Ophthalmol 1969;82:10-4
3 Little SA, Sruce AS. Repeatability of phenol-red and tear thinning time test for tear film function.Clin Exp Optom 1994;77:66-68
4 Little SA, Sruce AS. Repeatability of phenol-red and tear thinning time test for tear film function.Clin Exp Optom 1994;77:66-68